Adv. Theory Simul. 1, 1700008 (2018)
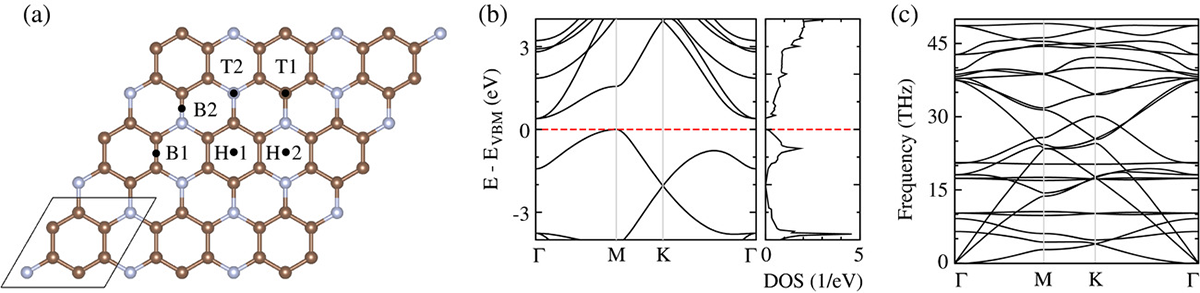
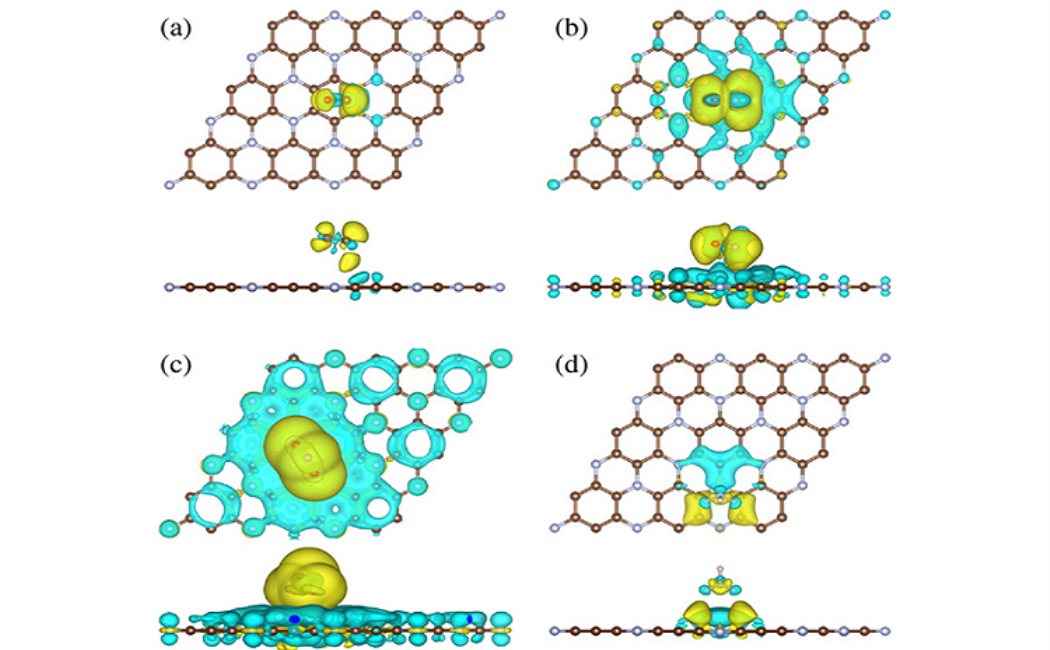
Using density functional theory with van der Waals dispersion correction, the adsorption behavior of common gaseous pollutants (CO, NO, NO2, and NH3) on monolayer C3N is investigated. The adsorption sites and energies, binding distances, charge transfers, and electronic band structures are calculated to understand the influence of the adsorbed molecules on the transport properties of monolayer C3N. The current-voltage characteristics are calculated using the nonequilibrium Green's function formalism. It turns out that all investigated molecules are physisorbed on monolayer C3N and that NO and NO2 gases can be sensed with high sensitivity. The recovery time of the sensor is found to be outstanding in the case of NO sensing (2.4 μs at room temperature) and competitive to other materials in the case of NO2 sensing.